DOI: 10.1007/s13361-017-1837-2
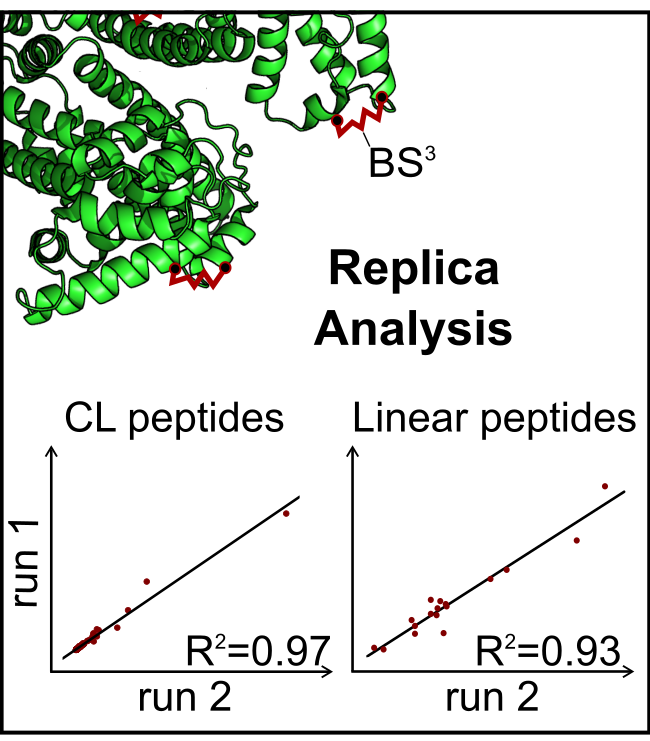
Abstract. Quantitative cross-linking/mass spectrometry (QCLMS) is an emerging approach to study conformational changes of proteins and multi-subunit complexes. Distinguishing protein conformations requires reproducibly identifying and quantify- ing cross-linked peptides. Here we analyzed the variation between multiple cross- linking reactions using bis[sulfosuccinimidyl] suberate (BS3)-cross-linked human serum albumin (HSA) and evaluated how reproducible cross-linked peptides can be identified and quantified by LC-MS analysis. To make QCLMS accessible to a broader research community, we developed a workflow that integrates the established software tools MaxQuant for spectra preprocessing, Xi for cross-linked peptide identification, and finally Skyline for quantification (MS1 filtering). Out of the
221 unique residue pairs identified in our sample, 124 were subsequently quantified across 10 analyses with coefficient of variation (CV) values of 14% (injection replica) and 32% (reaction replica). Thus our results demonstrate that the reproducibility of QCLMS is in line with the reproducibility of general quantitative proteomics and we establish a robust workflow for MS1-based quantitation of cross-linked peptides.