https://doi.org/10.1021/acs.analchem.1c04373
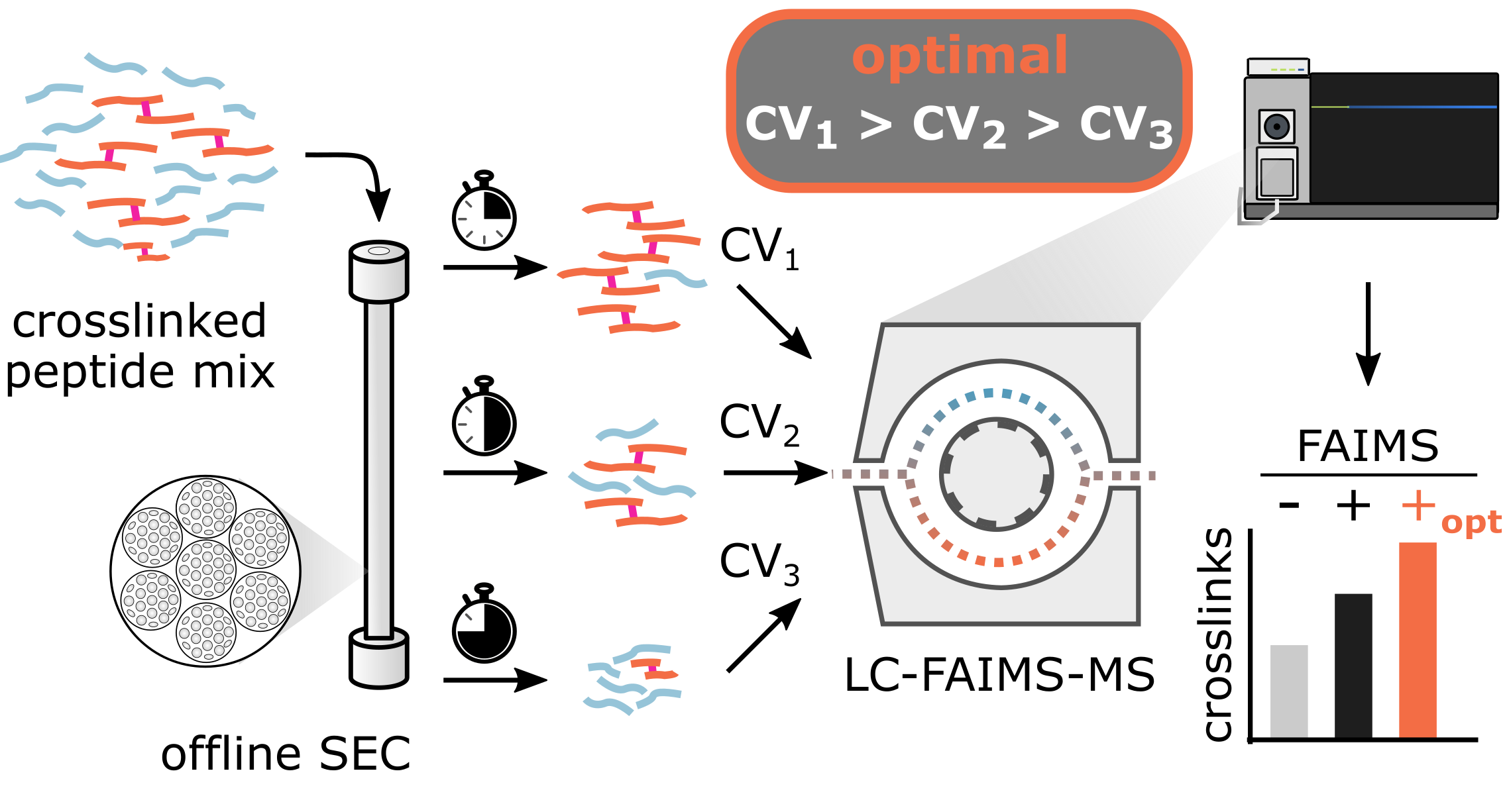
Ion mobility spectrometry shows great promise to tackle analytically challenging research questions by adding another separation dimension to liquid chromatography – mass spectrometry. The understanding of how analyte properties influence ion mobility has increased through recent studies but no clear rationale for the design of customized experimental settings has emerged. Here, we leverage machine learning to deepen our understanding of field asymmetric-waveform ion-mobility spectrometry (FAIMS) for the analysis of crosslinked peptides. Knowing that predominantly m/z, then size and charge state of an analyte influences the separation, we found ideal compensation voltages correlating with size-exclusion chromatography (SEC) fraction number. The effect of this relationship on analytical depth can be substantial as exploiting it allowed us to almost double unique residue pair detections in a proteome-wide crosslinking experiment. Other applications involving liquid- and gas-phase separation may also benefit from considering such parameter dependencies.